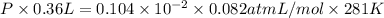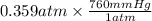Chemistry, 26.06.2019 04:20 silviamgarcia
The vapor pressure of liquid acetone, ch3coch3, is 100 mm hg at 281 k. a 6.06e-2 g sample of liquid ch3coch3 is placed in a closed, evacuated 360. ml container at a temperature of 281 k. calculate what the ideal gas pressure would be in the container if all of the liquid acetone evaporated.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 16:30
In chile, the deepest earthquake occurred at 61.7°w longitude at a depth of 540 km. if the rocks at the focus began subducting 10 million years ago and are now 1000 km from their original position, what is the average rate of subduction in cm/yr?
Answers: 1
You know the right answer?
The vapor pressure of liquid acetone, ch3coch3, is 100 mm hg at 281 k. a 6.06e-2 g sample of liquid...
Questions

Mathematics, 11.05.2021 01:00

Chemistry, 11.05.2021 01:00



Mathematics, 11.05.2021 01:00

English, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00




Biology, 11.05.2021 01:00


Chemistry, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00

Biology, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00


Biology, 11.05.2021 01:00

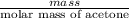
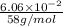
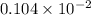 mole
mole