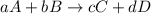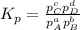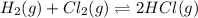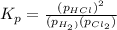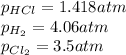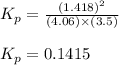Chemistry, 26.06.2019 03:20 elopezhilario6339
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibrium. the equilibrium pressure of hcl is found to be 1.418 atm. calculate kp for the reaction at this temperature. h2(g) + cl2(g) < => 2 hcl(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1
You know the right answer?
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibri...
Questions





Social Studies, 21.06.2019 15:30

History, 21.06.2019 15:30

Chemistry, 21.06.2019 15:30











Chemistry, 21.06.2019 15:30

Physics, 21.06.2019 15:30

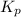 for the given chemical reaction is 0.1415
for the given chemical reaction is 0.1415