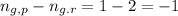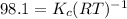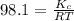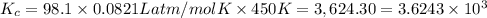Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl3(g)+cl2(g)⇌pcl5(g). a 7.5-l gas vessel is charged with a mixture of pcl3(g) and cl2(g), which is allowed to equilibrate at 450 k. at equilibrium the partial pressures of the three gases are ppcl3 = 0.125atm , pcl2 = 0.155atm , and ppcl5 = 1.90atm kp= 98.1 what is kc?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: pcl3(g)+cl2...
Questions

Chemistry, 05.11.2019 08:31



Physics, 05.11.2019 08:31

History, 05.11.2019 08:31

History, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31

Chemistry, 05.11.2019 08:31


Business, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31


Chemistry, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31


Chemistry, 05.11.2019 08:31

Biology, 05.11.2019 08:31

Biology, 05.11.2019 08:31

Chemistry, 05.11.2019 08:31

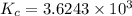 .
.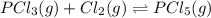
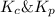 is given by:
is given by: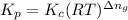
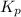 = Equilibrium constant in terms of partial pressure.=98.1
= Equilibrium constant in terms of partial pressure.=98.1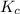 = Equilibrium constant in terms of concentration =?
= Equilibrium constant in terms of concentration =?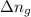 = Difference between gaseous moles on product side and reactant side=
= Difference between gaseous moles on product side and reactant side=