
Chemistry, 28.01.2020 01:31 JessTaylr04
Aself-contained underwater breathing apparatus (scuba) uses canisters containing potassium superoxide. the superoxide consumes the co2 exhaled by a person and replaces it with oxygen. 4 ko2(s) + 2 co2(g) n 2 k2co3(s) + 3 o2(g) what mass of ko2, in grams, is required to react with 8.90 l of co2 at 22.0 °c and 767 mm hg

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3


Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

You know the right answer?
Aself-contained underwater breathing apparatus (scuba) uses canisters containing potassium superoxid...
Questions



Chemistry, 19.01.2021 22:40

Geography, 19.01.2021 22:40




Mathematics, 19.01.2021 22:40


Advanced Placement (AP), 19.01.2021 22:40



Social Studies, 19.01.2021 22:40

Chemistry, 19.01.2021 22:40




English, 19.01.2021 22:40


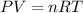
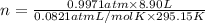
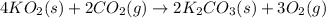
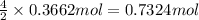 of potassium superoxide.
of potassium superoxide.

