Chemistry, 23.01.2020 04:31 caitlynnstokes
In an electroplating process, copper (ionic charge +2e, atomic weight 63.6 g/mol) is deposited using a current of 10.0 a. what mass of copper is deposited in 10.0 minutes? avogadro's number is 6.022 × 1023 molecules/mol and e = 1.60 × 10-19 c.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
In an electroplating process, copper (ionic charge +2e, atomic weight 63.6 g/mol) is deposited using...
Questions




Business, 18.07.2019 13:30

Social Studies, 18.07.2019 13:30



Mathematics, 18.07.2019 13:30

Biology, 18.07.2019 13:30


Mathematics, 18.07.2019 13:30


Mathematics, 18.07.2019 13:30



Mathematics, 18.07.2019 13:30




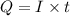
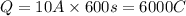
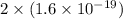
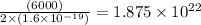 atoms
atoms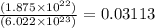 moles
moles