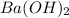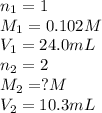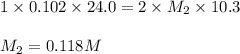Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3
You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.102 m solution of perc...
Questions

English, 15.07.2019 10:30

English, 15.07.2019 10:30


Chemistry, 15.07.2019 10:30

Mathematics, 15.07.2019 10:30



Mathematics, 15.07.2019 10:30

Mathematics, 15.07.2019 10:30

Mathematics, 15.07.2019 10:30

Mathematics, 15.07.2019 10:30




English, 15.07.2019 10:30

English, 15.07.2019 10:30

English, 15.07.2019 10:30


Chemistry, 15.07.2019 10:30

History, 15.07.2019 10:30

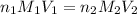
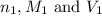 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 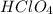
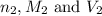 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is