Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
The equilibrium constant, kc, for the following reaction is 1.29×10-2 at 600 k. cocl2(g) co(g) + cl2...
Questions





Mathematics, 24.02.2021 19:40

Mathematics, 24.02.2021 19:40




Chemistry, 24.02.2021 19:40

Mathematics, 24.02.2021 19:40

Mathematics, 24.02.2021 19:40






Mathematics, 24.02.2021 19:40


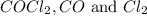 are, 0.2646, 0.0584 and 0.0584.
are, 0.2646, 0.0584 and 0.0584.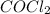 = 0.323 mole
= 0.323 mole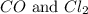 be, 'x'. So,
be, 'x'. So,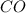 = x M
= x M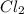 = x M
= x M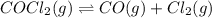
![K_c=\frac{[CO][Cl_2]}{[COCl_2]}](/tpl/images/0436/7314/59c52.png)