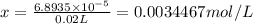Chemistry, 20.01.2020 18:31 DeonDub3106
Titration of a 20.0-ml sample of acid rain required 1.7 ml of 0.0811 m naoh to reach the end point. if we assume that the acidity of the rain is due to the presence of sulfuric acid, what was the concentration of sulfuric acid in this sample of rain?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 3

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
Titration of a 20.0-ml sample of acid rain required 1.7 ml of 0.0811 m naoh to reach the end point....
Questions

Mathematics, 11.01.2021 18:30

Mathematics, 11.01.2021 18:30

Mathematics, 11.01.2021 18:30


Business, 11.01.2021 18:30

Mathematics, 11.01.2021 18:30



Mathematics, 11.01.2021 18:40

Mathematics, 11.01.2021 18:40

Social Studies, 11.01.2021 18:40




Mathematics, 11.01.2021 18:40




Physics, 11.01.2021 18:40

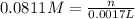
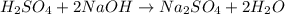
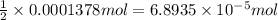 of sulfuric acid.
of sulfuric acid.