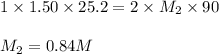Chemistry, 28.01.2020 11:31 covergurllaa
Avolume of 90.0 ml of aqueous potassium hydroxide (koh) was titrated against a standard solution of sulfuric acid (h2so4). what was the molarity of the koh solution if 25.2 ml of 1.50 m h2so4 was needed? the equation is 2koh(aq)+h2so4(aq)→k2so4(aq)+2h2o(l )

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Avolume of 90.0 ml of aqueous potassium hydroxide (koh) was titrated against a standard solution of...
Questions


Physics, 31.01.2020 09:01


Biology, 31.01.2020 09:01


Biology, 31.01.2020 09:01


World Languages, 31.01.2020 09:01

Mathematics, 31.01.2020 09:01




Mathematics, 31.01.2020 09:01

History, 31.01.2020 09:01

English, 31.01.2020 09:01

History, 31.01.2020 09:02




Chemistry, 31.01.2020 09:02

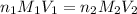
 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 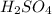
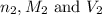 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.