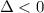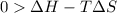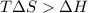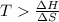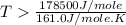For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variations in thermodynamic values for different sources, be sure to use the given values in calculating your answer.): δh∘rxn 178.5kj/mol δs∘rxn 161.0j/(mol⋅k) calculate the temperature in kelvins above which this reaction is spontaneous.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1


Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

You know the right answer?
For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variat...
Questions

Mathematics, 11.02.2021 14:00


Computers and Technology, 11.02.2021 14:00


Biology, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

English, 11.02.2021 14:00



Chemistry, 11.02.2021 14:00



Arts, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00


Chemistry, 11.02.2021 14:00

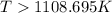
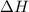 = 178.5 KJ/mole = 178500 J/mole
= 178.5 KJ/mole = 178500 J/mole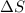 = 161.0 J/mole.K
= 161.0 J/mole.K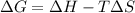
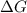 is negative or we can say that the value of
is negative or we can say that the value of