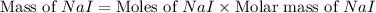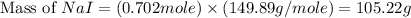Chemistry, 15.11.2019 14:31 ejfleck3655
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solution containing sodium iodide. 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) how many grams of sodium iodide, nai, nai, must be used to produce 89.1 g89.1 g of iodine, i2? i2? mass: g nai

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solu...
Questions




Mathematics, 07.07.2020 22:01


Biology, 07.07.2020 22:01

History, 07.07.2020 22:01


Computers and Technology, 07.07.2020 22:01

Mathematics, 07.07.2020 22:01

Computers and Technology, 07.07.2020 22:01


History, 07.07.2020 22:01


Mathematics, 07.07.2020 22:01


English, 07.07.2020 22:01



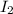 = 89.1 g
= 89.1 g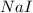 = 149.89 g/mole
= 149.89 g/mole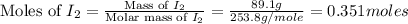
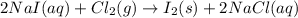
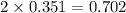 moles of
moles of