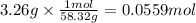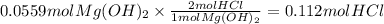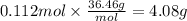Chemistry, 16.10.2019 23:30 wiljoystoltz253
Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily hcl, according to the reaction mg(oh)2(aq)+2hcl(aq)→2h2o(l)+mgcl2( aq) what mass of hcl, in grams, is neutralized by a dose of milk of magnesia containing 3.26 g of mg(oh)2? express the mass in grams to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1


Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1
You know the right answer?
Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily...
Questions

SAT, 24.01.2021 05:40


Mathematics, 24.01.2021 05:40


History, 24.01.2021 05:40

Physics, 24.01.2021 05:40

Mathematics, 24.01.2021 05:40

Physics, 24.01.2021 05:40

Physics, 24.01.2021 05:40

Mathematics, 24.01.2021 05:40



Mathematics, 24.01.2021 05:40


Mathematics, 24.01.2021 05:40



Mathematics, 24.01.2021 05:40

Mathematics, 24.01.2021 05:40