
Chemistry, 13.10.2019 21:30 nidiavega2009
You start with 0.050 moles of ammonia in 500. ml of water. the equilibrium constant keq is 1.8 × 10–5. what is the ph of this solution at equilibrium? show work!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1


Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
You start with 0.050 moles of ammonia in 500. ml of water. the equilibrium constant keq is 1.8 × 10–...
Questions

Mathematics, 26.03.2021 17:40

History, 26.03.2021 17:40


Mathematics, 26.03.2021 17:40


Mathematics, 26.03.2021 17:40

Mathematics, 26.03.2021 17:40



Mathematics, 26.03.2021 17:40

Biology, 26.03.2021 17:40




Mathematics, 26.03.2021 17:40


History, 26.03.2021 17:40



Mathematics, 26.03.2021 17:40

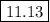
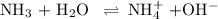
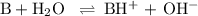
![\text{[B]} = \dfrac{\text{moles}}{\text{litres}} = \dfrac{\text{0.050 mol}}{\text{0.500 L}} = \text{0.100 mol/L}](/tpl/images/0316/7651/145d4.png)
![K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.100 - x} = 1.8 \times 10^{-5}](/tpl/images/0316/7651/4767e.png)
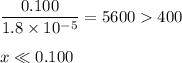
![\dfrac{x^{2}}{0.100} = 1.8 \times 10^{-5}\\\\x^{2} = 0.100 \times 1.8 \times 10^{-5}\\\\x^{2} = 1.80 \times 10^{-6}\\\\x = \sqrt{1.80 \times 10^{-6}}\\\\x = \text{[OH]}^{-} = 1.34 \times 10^{-3} \text{ mol/L}](/tpl/images/0316/7651/c562e.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(1.34 \times 10^{-3}) = 2.87\\\\\text{pH} = 14.00 - \text{pOH} = 14.00 - 2.87 = \mathbf{11.13}\\\\\text{The pH of the solution at equilibrium is } \boxed{\mathbf{11.13}}](/tpl/images/0316/7651/2b390.png)



