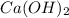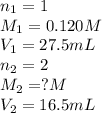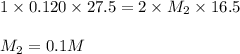Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hyd...
Questions

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

History, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

English, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

Mathematics, 18.09.2020 05:01

English, 18.09.2020 05:01

English, 18.09.2020 05:01

Biology, 18.09.2020 05:01

Biology, 18.09.2020 05:01

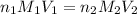
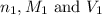 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 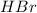
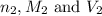 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is