
Chemistry, 22.06.2019 00:00 reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1


Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) addin...
Questions

Mathematics, 04.11.2021 08:10

Mathematics, 04.11.2021 08:10






Mathematics, 04.11.2021 08:10


Mathematics, 04.11.2021 08:10

Health, 04.11.2021 08:10

English, 04.11.2021 08:10

Social Studies, 04.11.2021 08:10

Mathematics, 04.11.2021 08:10

Mathematics, 04.11.2021 08:10





Mathematics, 04.11.2021 08:10

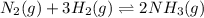
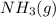 will shift the given equilibrium system to the left.
will shift the given equilibrium system to the left.

