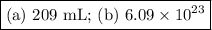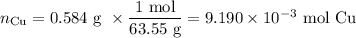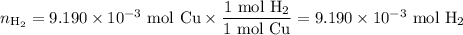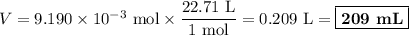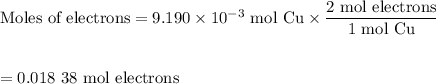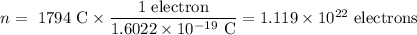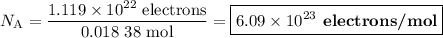An acidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. (a) what is the gas produced at the cathode and what is its volume at stp? name of gas volume of gas webassign will check your answer for the correct number of significant figures. l (b) given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3
You know the right answer?
An acidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused...
Questions


Chemistry, 30.08.2019 07:00

Mathematics, 30.08.2019 07:00

Mathematics, 30.08.2019 07:00

Mathematics, 30.08.2019 07:00



Health, 30.08.2019 07:00

English, 30.08.2019 07:00



Mathematics, 30.08.2019 07:00



Mathematics, 30.08.2019 07:00



History, 30.08.2019 07:00