
Chemistry, 22.06.2019 18:30 chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a...
Questions




English, 31.03.2020 20:14

Mathematics, 31.03.2020 20:14

Chemistry, 31.03.2020 20:14



Mathematics, 31.03.2020 20:14

Mathematics, 31.03.2020 20:14

English, 31.03.2020 20:14



Mathematics, 31.03.2020 20:15

Mathematics, 31.03.2020 20:15

Mathematics, 31.03.2020 20:15

Computers and Technology, 31.03.2020 20:15

Mathematics, 31.03.2020 20:15


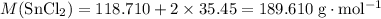 .
.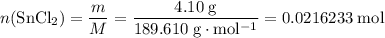 .
.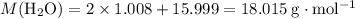 .
.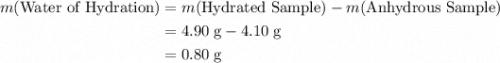 .
.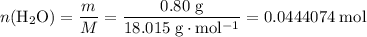 .
.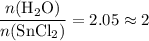 .
.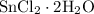 .
.

