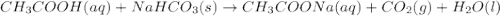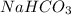Chemistry, 24.06.2019 15:20 janakibubbles7711
Vinegar is an aqueous solution of acetic acid (hch3coo). when vinegar reacts with dry baking soda (nahco3), the result is an aqueous solution of sodium acetate (nach3coo), liquid water, and carbon-dioxide gas, which bubbles out of the solution. which chemical equation correctly represents this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Vinegar is an aqueous solution of acetic acid (hch3coo). when vinegar reacts with dry baking soda (n...
Questions




Business, 24.03.2020 02:53



Biology, 24.03.2020 02:53


Biology, 24.03.2020 02:54

Mathematics, 24.03.2020 02:54



Mathematics, 24.03.2020 02:54



Physics, 24.03.2020 02:54


Chemistry, 24.03.2020 02:54