
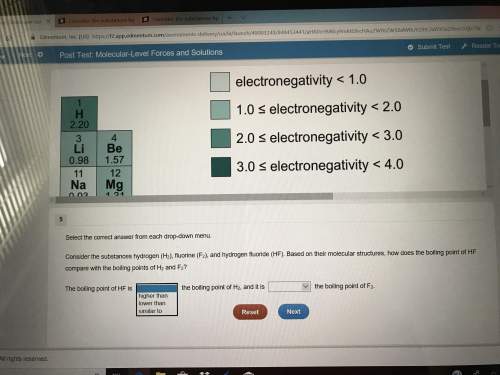
Plz don’t have much time left(picture included)select the correct answer from each drop-down menu. consider the substances hydrogen (h2), fluorine (f2), and hydrogen fluoride (hf). based on their molecular structures, how does the boiling point of hf compare with the boiling points of h2 and f2? the boiling point of hf is the boiling point of h2, and it is the boiling point of f2second set of opinions is the same

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

You know the right answer?
Plz don’t have much time left(picture included)select the correct answer from each drop-down menu....
Questions

History, 07.09.2020 14:01

English, 07.09.2020 14:01




Mathematics, 07.09.2020 14:01

English, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01


Mathematics, 07.09.2020 14:01

Social Studies, 07.09.2020 14:01


Geography, 07.09.2020 14:01

Biology, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01

Biology, 07.09.2020 14:01

English, 07.09.2020 14:01

Mathematics, 07.09.2020 14:01

Business, 07.09.2020 14:01



