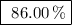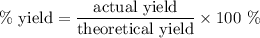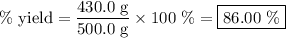Chemistry, 25.06.2019 08:00 saadizak7098
Achemical company produces ammonia using the following reaction: n2 + 3h2 → 2nh3they run a reaction meant to fill an order for a customer who would like to purchase 500.0g of ammonia. when the reaction is complete, the company finds they produced only 430.0g of ammonia. what it their percent yield for that reaction? 70.00%86.00%116.3%93.00%

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2


You know the right answer?
Achemical company produces ammonia using the following reaction: n2 + 3h2 → 2nh3they run a reaction...
Questions

History, 17.11.2019 16:31


Social Studies, 17.11.2019 16:31





Biology, 17.11.2019 16:31



English, 17.11.2019 16:31

Mathematics, 17.11.2019 16:31


History, 17.11.2019 16:31

Mathematics, 17.11.2019 16:31



Chemistry, 17.11.2019 16:31


History, 17.11.2019 16:31