
Chemistry, 25.06.2019 19:50 summerdooleyu
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is produced. b. co2 concentration increases. c. the equilibrium is pushed in the direction of reactants. d. nothing
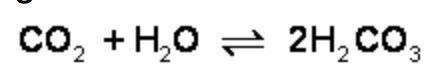

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is pro...
Questions


Mathematics, 15.02.2021 15:50

Social Studies, 15.02.2021 15:50

Geography, 15.02.2021 15:50

History, 15.02.2021 15:50

History, 15.02.2021 15:50

Social Studies, 15.02.2021 15:50

Mathematics, 15.02.2021 15:50

Business, 15.02.2021 15:50

Social Studies, 15.02.2021 15:50

German, 15.02.2021 15:50

English, 15.02.2021 15:50


Engineering, 15.02.2021 15:50





Mathematics, 15.02.2021 15:50

Physics, 15.02.2021 15:50



