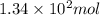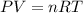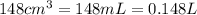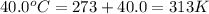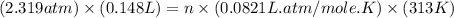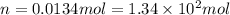Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
At 40.0°c, the pressure inside a nitrogen-filled tennis ball with a volume of 148 cm is 235 kpa. how...
Questions




Mathematics, 16.03.2022 14:00



Mathematics, 16.03.2022 14:00






Chemistry, 16.03.2022 14:00

Mathematics, 16.03.2022 14:00



Biology, 16.03.2022 14:00

Biology, 16.03.2022 14:00