
Chemistry, 18.11.2019 13:31 ansarishaheer2888
Given the balanced equation representing a reaction: 4al(s) + 3o2(g) → 2al2o3(s) how many moles of al(s) react completely with 4.50 moles of o2(g) to produce 3.00 moles of al2o3(s)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Given the balanced equation representing a reaction: 4al(s) + 3o2(g) → 2al2o3(s) how many moles of...
Questions

Biology, 23.07.2019 16:30


Biology, 23.07.2019 16:30



Chemistry, 23.07.2019 16:30











History, 23.07.2019 16:30


History, 23.07.2019 16:30

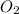 :
: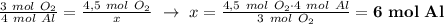
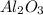 , keeping constant the proportion of both substances.
, keeping constant the proportion of both substances.

