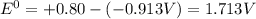Based on the sign of e cell, classify these reactions as spontaneous or non spontaneous as written.? assume standard conditions. ni^2+ (aq) + s^2- (aq) > + ni (s) s (s) (nonspontaneous)? pb^2+ (aq) +h2 (g) > pb (s) +2h^+ (aq) (nonspontaneous)? 2ag^+ (aq) + cr(s) > 2 ag (s) +cr^2+ (aq) (spontaneous? ) are these correct?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Based on the sign of e cell, classify these reactions as spontaneous or non spontaneous as written.?...
Questions

Mathematics, 25.02.2021 17:40

Engineering, 25.02.2021 17:40


Chemistry, 25.02.2021 17:40

Chemistry, 25.02.2021 17:40

Social Studies, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40




Mathematics, 25.02.2021 17:40

English, 25.02.2021 17:40

Chemistry, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40


Mathematics, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40

Mathematics, 25.02.2021 17:40

Spanish, 25.02.2021 17:40

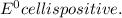
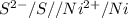
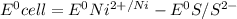
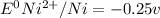
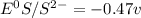
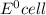 = - 0.25 - (-0.47) = 0.22 v
= - 0.25 - (-0.47) = 0.22 v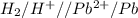
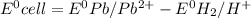
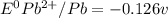
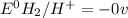
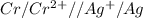
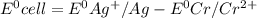
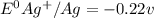
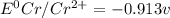
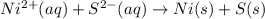 : non spontaneous
: non spontaneous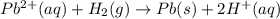 : non spontaneous
: non spontaneous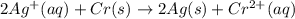 : spontaneous
: spontaneous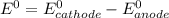
 are standard reduction potentials.
are standard reduction potentials.
![E^0_{[Ni^{2+}/Ni]}=-0.25V](/tpl/images/0340/4979/2bf3e.png)
![E^0_{[S^{2-}/S]}=0.407VV](/tpl/images/0340/4979/b9faa.png)
![E^0=E^0_{[Ni^{2+}/Ni]}- E^0_{[S^{2-}/S]}](/tpl/images/0340/4979/7165d.png)
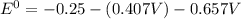
![E^0_{[Pb^{2+}/Pb]}=-0.13](/tpl/images/0340/4979/2e29f.png)
![E^0_{[H^{+}/H_2]}=0V](/tpl/images/0340/4979/311ce.png)
![E^0=E^0_{[Pb^{2+}/Pb]}- E^0_{[H^{+}/H_2]}](/tpl/images/0340/4979/1bbe8.png)
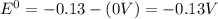
![E^0_{[Ag^{+}/Ag]}=+0.80V](/tpl/images/0340/4979/76e17.png)
![E^0_{[Cr^{2+}/Cr]}=-0.913V](/tpl/images/0340/4979/0a8f3.png)
![E^0=E^0_{[Ag^{+}/Ag]}- E^0_{[Cr^{2+}/Cr]}](/tpl/images/0340/4979/5f991.png)