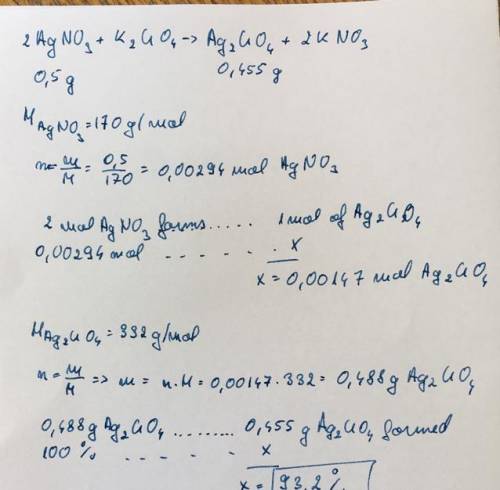Chemistry, 27.06.2019 07:20 maxi12312345
3. calculate the percent yield of silver chromate if 0.455 grams of silver chromate are producedfrom 0.500 grams of silver nitrate.2 agno3 + k2cro4 -> ag2 cro 4 + 2 kno3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
3. calculate the percent yield of silver chromate if 0.455 grams of silver chromate are producedfrom...
Questions


Mathematics, 02.12.2021 16:30

Social Studies, 02.12.2021 16:30

Mathematics, 02.12.2021 16:30

SAT, 02.12.2021 16:30




Biology, 02.12.2021 16:40

English, 02.12.2021 16:40


Spanish, 02.12.2021 16:40

Chemistry, 02.12.2021 16:40

English, 02.12.2021 16:40

Mathematics, 02.12.2021 16:40



English, 02.12.2021 16:40