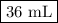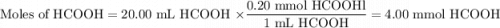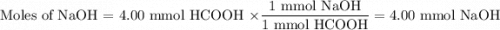You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you will titrate this with a 0.1105 m naoh. if you add 20.00 ml of hcooh to the beaker before titrating, approximately what volume of naoh will be required to reach the end point? view available hint(s) you are given a solution of (formic acid) with an approximate concentration of 0.20 and you will titrate this with a 0.1105 . if you add 20.00 of to the beaker before titrating, approximately what volume of will be required to reach the end point? 11.1 ml 20.0 ml 72.4 ml 36.2 ml

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 12:30
If you reacted 450 g of trimethylgallium with 300 g of arsine, what mass of gaas could you make?
Answers: 1

Chemistry, 23.06.2019 13:30
32p and 31p are two isotopes of phosphorus. compare the number if subatomic particles that are present in the atoms of these isotopes.
Answers: 1
You know the right answer?
You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you...
Questions






Mathematics, 20.09.2020 09:01

Chemistry, 20.09.2020 09:01

Arts, 20.09.2020 09:01

History, 20.09.2020 09:01


Chemistry, 20.09.2020 09:01


English, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01


English, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01