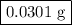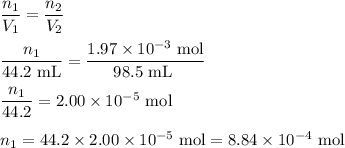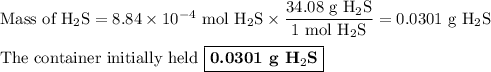Chemistry, 28.06.2019 05:50 dinarussell74
Hydrogen sulfide gas (h2s) is a highly toxic gas that is responsible for the smell of rotten eggs. the volume of a container of hydrogen sulfide is 44.2ml. after the addition of more hydrogen sulfide, the volume increases to 98.5ml under constant pressure and temperature. the container now holds 1.97×10−3mol of the gas. how many grams of hydrogen sulfide were in the container initially? give your answer in three significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2



Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1
You know the right answer?
Hydrogen sulfide gas (h2s) is a highly toxic gas that is responsible for the smell of rotten eggs. t...
Questions

Chemistry, 21.12.2019 21:31

Mathematics, 21.12.2019 21:31


Geography, 21.12.2019 21:31



Biology, 21.12.2019 21:31

Mathematics, 21.12.2019 21:31


Mathematics, 21.12.2019 21:31



Business, 21.12.2019 21:31


History, 21.12.2019 21:31

Mathematics, 21.12.2019 21:31

Biology, 21.12.2019 21:31

English, 21.12.2019 21:31

History, 21.12.2019 21:31