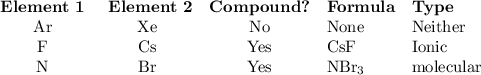Chemistry, 28.06.2019 05:50 deelashasharma
For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. if they will, then enter the chemical formula of the compound. if the elements will form more than one compound, enter the compound with the fewest total number of atoms you may assume all chemical bonds are single bonds, not double or triple bonds element #1 | element #2 | compound formed? | chemical formula ionic o molecular o neither argon xenon ionic o molecular o neither fluorine cesiumm ionic o molecular o neither nitrogen bromine

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
For each row in the table below, decide whether the pair of elements will form a molecular or ionic...
Questions


Mathematics, 02.03.2021 01:00

Computers and Technology, 02.03.2021 01:00



Mathematics, 02.03.2021 01:00

History, 02.03.2021 01:00



Mathematics, 02.03.2021 01:00







Mathematics, 02.03.2021 01:00

Business, 02.03.2021 01:00


Biology, 02.03.2021 01:00