Chemistry, 29.06.2019 16:00 avahrhey24
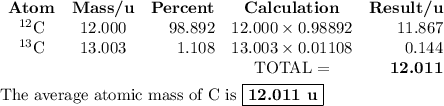
C-12 and c-13 are naturally-occurring isotopes of the element carbon. c-12 occurs 98.89% of the time and c-13 occurs 1.108% of the time. what calculation should be used to determine the atomic mass of this element?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
C-12 and c-13 are naturally-occurring isotopes of the element carbon. c-12 occurs 98.89% of the time...
Questions


History, 27.09.2019 22:00

Mathematics, 27.09.2019 22:00


English, 27.09.2019 22:00



History, 27.09.2019 22:00


Mathematics, 27.09.2019 22:00

Arts, 27.09.2019 22:00

Biology, 27.09.2019 22:00


History, 27.09.2019 22:00

History, 27.09.2019 22:00


History, 27.09.2019 22:00

Chemistry, 27.09.2019 22:00