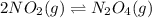Chemistry, 29.06.2019 21:50 dooboose15
In the reversible reaction: 2no2 (g) ⇌ n2o4 (g) the formation of dinitrogen tetroxide releases heat and the formation of nitrogen dioxide absorbs heat. if the reaction is at equilibrium and the temperature increases, what will the effect be? a. nitrogen dioxide absorbs heat and changes from gas to liquid. b. the equilibrium will shift so that there is more nitrogen dioxide. c. the equilibrium will shift so that there is more dinitrogen tetroxide. d. dinitrogen tetroxide absorbs heat and changes from gas to liquid.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
You know the right answer?
In the reversible reaction: 2no2 (g) ⇌ n2o4 (g) the formation of dinitrogen tetroxide releases heat...
Questions


Physics, 22.06.2019 04:00





Geography, 22.06.2019 04:00





Mathematics, 22.06.2019 04:00

World Languages, 22.06.2019 04:00

World Languages, 22.06.2019 04:00




Health, 22.06.2019 04:00