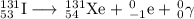Some radioactive nuclides have very short half-lives, for example, i-31 has a half-life of approximately 8 days. pu-234, by comparison has a half-life of 24,000 years. explain why both of these examples are dangerous, even though their half-lives are very different. be sure to describe the different major types of radiation, and their hazards. (radioactive decay and half-life)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
Some radioactive nuclides have very short half-lives, for example, i-31 has a half-life of approxima...
Questions

Mathematics, 17.09.2019 00:00

Social Studies, 17.09.2019 00:00



Biology, 17.09.2019 00:00


History, 17.09.2019 00:00


Mathematics, 17.09.2019 00:00

Chemistry, 17.09.2019 00:00





Mathematics, 17.09.2019 00:00


English, 17.09.2019 00:00