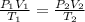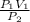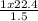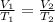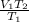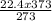Acontainer holds 22.4 l of gas at 1.00 atm and 0.0˚c. a. convert 0.0˚c to kelvin. b. state the combined gas law. c. if the pressure increases to 1.50 atm and the temperature doesn’t change, calculate the new volume. d. if the temperature increases to 100.0 ˚c and the pressure doesn’t change, calculate the new volume.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2


Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
Acontainer holds 22.4 l of gas at 1.00 atm and 0.0˚c. a. convert 0.0˚c to kelvin. b. state the combi...
Questions


Geography, 30.10.2020 19:50

Mathematics, 30.10.2020 19:50

Biology, 30.10.2020 19:50


Mathematics, 30.10.2020 19:50



Chemistry, 30.10.2020 19:50

Mathematics, 30.10.2020 19:50



Mathematics, 30.10.2020 19:50

Biology, 30.10.2020 19:50

English, 30.10.2020 19:50

Chemistry, 30.10.2020 19:50


Computers and Technology, 30.10.2020 19:50

Mathematics, 30.10.2020 19:50

English, 30.10.2020 19:50