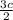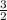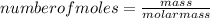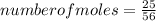Chemistry, 30.06.2019 06:40 saucydolphin20p6x92l
Consider the following reaction: iron (s) + chlorine (g) à iron (iii) chloride a. write the balanced chemical equation. b. 25.0 g of iron reacts with excess chlorine gas. a. calculate the moles of iron reactant. b. calculate the moles of iron (iii) chloride. c. calculate the mass of iron (iii) chloride.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Consider the following reaction: iron (s) + chlorine (g) à iron (iii) chloride a. write the balance...
Questions



Biology, 05.10.2021 14:00


Mathematics, 05.10.2021 14:00

World Languages, 05.10.2021 14:00



Physics, 05.10.2021 14:00


English, 05.10.2021 14:00




Mathematics, 05.10.2021 14:00

SAT, 05.10.2021 14:00

Biology, 05.10.2021 14:00

Mathematics, 05.10.2021 14:00

Mathematics, 05.10.2021 14:00