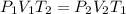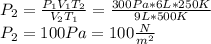Chemistry, 30.06.2019 07:20 redrhino27501
At a temperature of 500 kelvins, 6 liters of an ideal gas had a pressure of 300 newtons per square meter. if the temperature was reduced to 250 kelvins, and the volume raised to 9 liters, what was the resulting pressure ? (a) 100 newtons/m^2 (b) 150 newtons/m^2 (c) 450 newtons/m^2 (d) 900 newtons/m^2 (e) none of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
At a temperature of 500 kelvins, 6 liters of an ideal gas had a pressure of 300 newtons per square m...
Questions


Mathematics, 08.07.2019 15:30


Mathematics, 08.07.2019 15:30


Mathematics, 08.07.2019 15:30

Spanish, 08.07.2019 15:30

Mathematics, 08.07.2019 15:30

Mathematics, 08.07.2019 15:30

Mathematics, 08.07.2019 15:30



Social Studies, 08.07.2019 15:30

Social Studies, 08.07.2019 15:30


Mathematics, 08.07.2019 15:30

Biology, 08.07.2019 15:30

Mathematics, 08.07.2019 15:30


Mathematics, 08.07.2019 15:30