Chemistry, 04.01.2020 14:31 battlemarshmell
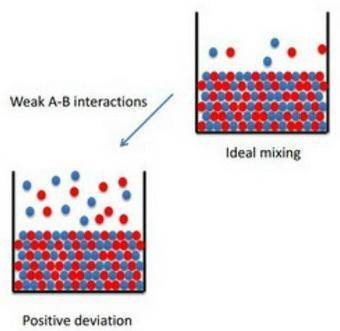
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 torr with an equimolar amount of a liquid with a vapor pressure of 200 torr. the resulting solution would be predicted to have a vapor pressure of 150 torr if it behaved ideally. if, however, the interactions between the different components are not similar we can see positive or negative deviations from the calculated vapor pressure. an actual vapor pressure greater than that predicted by raoult's law is said to be a positive deviation and an actual vapor pressure lower than that predicted by raoult's law is a negative deviation. part a imagine a solution of two liquids in which the molecules interact less favorably than they do in the individual liquids. will this solution deviate positively from, deviate negatively from, or ideally follow raoult's law? '

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 09:10
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1

Chemistry, 23.06.2019 10:30
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
You know the right answer?
How to apply raoult's law to real solutions consider mixing a liquid with a vapor pressure of 100 to...
Questions

English, 09.12.2021 04:00

SAT, 09.12.2021 04:00


English, 09.12.2021 04:00

SAT, 09.12.2021 04:00

Biology, 09.12.2021 04:00


Mathematics, 09.12.2021 04:00

English, 09.12.2021 04:00


Mathematics, 09.12.2021 04:00

Social Studies, 09.12.2021 04:00

English, 09.12.2021 04:00




Biology, 09.12.2021 04:00



Social Studies, 09.12.2021 04:00