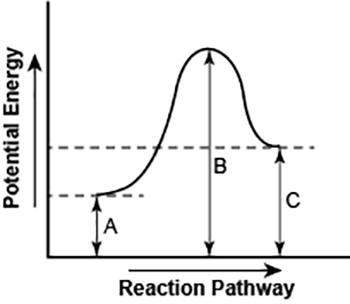
the diagram shows the potential energy changes for a reaction pathway.

Question:
the diagram shows the potential energy changes for a reaction pathway.
part 1: does the diagram illustrate an endothermic or an exothermic reaction? give reasons in support of your answer.
part 2: describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.
part 1: the diagram illustrates an endothermic reaction as the products has a higher potential energy than the reactants do. there is a positive slope of the diagram and there is enough energy to meet the activation energy requirement.
part 2: you can determine the total change in enthalpy and activation energy from the diagram by the potential energy of the reactants. if the reactants have a high potential energy, then the enthalpy is also high, and if the reactants have a low potential energy, then the enthalpy is low. you can determine if the diagram is positive or negative by knowing if its an endothermic or exothermic reaction. an endothermic reaction is positive because the products are higher than the reactants and a exothermic reaction is negative because the reactants are higher than the products.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2



Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
Question:
the diagram shows the potential energy changes for a reaction pathway.
the diagram shows the potential energy changes for a reaction pathway.
Questions




Physics, 02.05.2021 01:10



English, 02.05.2021 01:10



Law, 02.05.2021 01:10

Chemistry, 02.05.2021 01:10


Mathematics, 02.05.2021 01:10


Chemistry, 02.05.2021 01:10

Spanish, 02.05.2021 01:10


Biology, 02.05.2021 01:10


Chemistry, 02.05.2021 01:10



