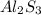Chemistry, 20.10.2019 09:50 strange5eyes
The formula of aluminium sulphide is al₂s₃. explain why the formula has a ratio of two aluminium ions for every three sulphide ions.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
The formula of aluminium sulphide is al₂s₃. explain why the formula has a ratio of two aluminium ion...
Questions

Social Studies, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50

Business, 25.08.2019 08:50


Mathematics, 25.08.2019 08:50


History, 25.08.2019 08:50

Chemistry, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50

History, 25.08.2019 08:50







Mathematics, 25.08.2019 09:00