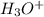Examine the following reaction.
hbrwater—→h++br−
what process is being shown...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Questions


English, 16.01.2020 20:31


Mathematics, 16.01.2020 20:31

Mathematics, 16.01.2020 20:31



Mathematics, 16.01.2020 20:31


Biology, 16.01.2020 20:31

English, 16.01.2020 20:31

English, 16.01.2020 20:31




History, 16.01.2020 20:31


Mathematics, 16.01.2020 20:31

History, 16.01.2020 20:31

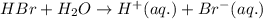
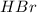 is loosing a proton, thus it is considered as an acid and the reaction is acid dissociation.
is loosing a proton, thus it is considered as an acid and the reaction is acid dissociation.