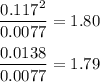Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1

Chemistry, 23.06.2019 07:30
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the...
Questions

Mathematics, 02.09.2020 17:01

Mathematics, 02.09.2020 17:01

Mathematics, 02.09.2020 17:01

Business, 02.09.2020 17:01

Mathematics, 02.09.2020 17:01



Mathematics, 02.09.2020 17:01


Mathematics, 02.09.2020 17:01

English, 02.09.2020 17:01


Chemistry, 02.09.2020 17:01


English, 02.09.2020 17:01

Biology, 02.09.2020 17:01

Health, 02.09.2020 17:01



![\boxed{\text{[PCl$_{5}$] = 0.0077 mol/L; [PCl$_{3}$] = [Cl$_{2}$] = 0.117 mol/L}}](/tpl/images/0449/9090/348ff.png)
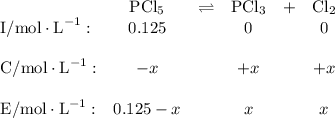
![K_{\text{c}} = \dfrac{\text{[PCl$_3$][Cl$_2$]}}{\text{[PCl$_5$]}} = \dfrac{x^{2}}{0.125-x} = 1.80](/tpl/images/0449/9090/2dff3.png)
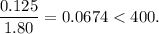
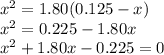
![\boxed{\textbf{[PCl$_{5}$] = 0.0077 mol/L; [PCl$_{3}$] = [Cl$_{2}$] = 0.117 mol/L}}](/tpl/images/0449/9090/a2840.png)