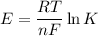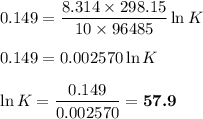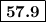Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
From standard reduction potentials, calculate the equilibrium constant at 25 ∘c for the reaction 2mn...
Questions






Mathematics, 21.05.2021 20:00

History, 21.05.2021 20:00



Mathematics, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00

World Languages, 21.05.2021 20:00



Mathematics, 21.05.2021 20:00


Mathematics, 21.05.2021 20:00

Mathematics, 21.05.2021 20:00

History, 21.05.2021 20:00