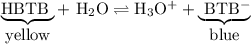Chemistry, 04.02.2020 12:47 bchagnard2122
Equilibrium lab analysis questions: use what you learned in this lab to answer the following questions in complete sentences. 1. bromthymol blue, btb is an acid which has a hydrogen ion, h+. for purposes of this question you will write it hbtb. hbtb ionizes in water to produce hydrogen ion, h+, and bromthymol blue ion, btb-.a) what color appeared when you added hcl to btb? b) what color appeared when you added naoh? c) explain the shift in equilibrium in terms of le chatelier's principle

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 11:00
Just on number 2 (all parts), and if you do answer explain in detail
Answers: 3
You know the right answer?
Equilibrium lab analysis questions: use what you learned in this lab to answer the following questi...
Questions





Mathematics, 05.11.2020 19:10



Computers and Technology, 05.11.2020 19:10

Biology, 05.11.2020 19:10

History, 05.11.2020 19:10

Mathematics, 05.11.2020 19:10






Mathematics, 05.11.2020 19:10

Arts, 05.11.2020 19:10