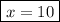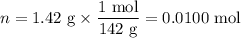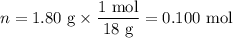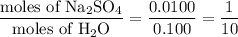Chemistry, 20.01.2020 08:31 mercedesamatap21hx0
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (molar mass 18 g) is driven off. the mass of the anhydrous na2so4 (s) (molar mass 142 g) that remains is 1.42g. the value of x in the hydrate is

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1


Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (mo...
Questions

English, 26.04.2020 06:43

Mathematics, 26.04.2020 06:43


Biology, 26.04.2020 06:44


History, 26.04.2020 06:44

Arts, 26.04.2020 06:44

Health, 26.04.2020 06:44



Mathematics, 26.04.2020 06:44

Mathematics, 26.04.2020 06:44

History, 26.04.2020 06:44


English, 26.04.2020 06:45

Mathematics, 26.04.2020 06:45



Computers and Technology, 26.04.2020 06:46

Mathematics, 26.04.2020 06:46