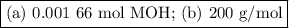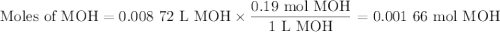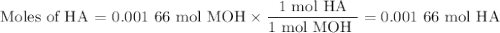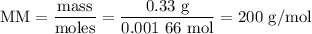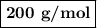Chemistry, 20.10.2019 06:50 goldenarrow
Using the concentration of the base and the volume of the base used, calculate the moles of the base used in the titration. then, using the mass of the acid, determine the molar mass of the acid.
data:
concentration of the base(naoh)= 0.19 m
volume of the base used= 8.72 ml
mass of the acid(unknown)= 0.33 g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Using the concentration of the base and the volume of the base used, calculate the moles of the base...
Questions


Mathematics, 04.10.2020 06:01


Mathematics, 04.10.2020 06:01





Social Studies, 04.10.2020 06:01


Biology, 04.10.2020 06:01

Mathematics, 04.10.2020 06:01

English, 04.10.2020 06:01





Social Studies, 04.10.2020 06:01

Mathematics, 04.10.2020 06:01

Mathematics, 04.10.2020 06:01