

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3


Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
What is the molarity of a solution containing 5 moles of hcl in 2.5 l of solution? be sure to inclu...
Questions


Mathematics, 19.12.2019 03:31

Mathematics, 19.12.2019 03:31

Mathematics, 19.12.2019 03:31


Geography, 19.12.2019 03:31


Mathematics, 19.12.2019 03:31

Social Studies, 19.12.2019 03:31

Mathematics, 19.12.2019 03:31

Mathematics, 19.12.2019 03:31

Physics, 19.12.2019 03:31

History, 19.12.2019 03:31


Mathematics, 19.12.2019 03:31





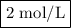
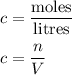
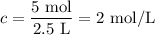
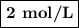 .
.


