
Chemistry, 09.10.2019 05:00 aleilyg2005
Given the following thermodynamic data, calculate the lattice energy of licl:
delta; h°f[licl(s)] = -409 kj/mol
δh°sublimation [li] = 161 kj/mol
bond energy [cl-cl] = 243 kj/mol
ie1 (li) = 520 kj/mol
ea1 (cl) = -349 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 05:30
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b.cns c. ans d. pns
Answers: 1
You know the right answer?
Given the following thermodynamic data, calculate the lattice energy of licl:
delta; h...
delta; h...
Questions






History, 05.04.2020 05:00

Mathematics, 05.04.2020 05:00


Social Studies, 05.04.2020 05:00


Mathematics, 05.04.2020 05:00


Mathematics, 05.04.2020 05:00

English, 05.04.2020 05:00

Biology, 05.04.2020 05:00

Mathematics, 05.04.2020 05:00



Mathematics, 05.04.2020 05:00

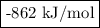
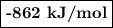 .
.

