

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
What is the molarity of a solution made by dissolving 5.5 g of ki in enough water to make 150 ml of...
Questions

Biology, 29.01.2021 22:00

Mathematics, 29.01.2021 22:00

Social Studies, 29.01.2021 22:00

Health, 29.01.2021 22:00



Physics, 29.01.2021 22:00


Arts, 29.01.2021 22:00

Mathematics, 29.01.2021 22:00


English, 29.01.2021 22:00


Mathematics, 29.01.2021 22:00


Social Studies, 29.01.2021 22:00




Advanced Placement (AP), 29.01.2021 22:00



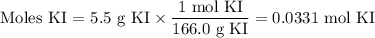
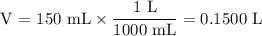
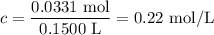
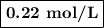 .
.

