
The requirement that a reversible reaction be at equilibrium is that:
- the concentrations on the two sides of the arrow be equal
- the velocity for the forward reaction equal that of the reverse reaction
- there will be as many molecules of the substances on one side of the arrow as there are molecules of the substances on the other side
- the moles of products will equal the moles of reactants

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
The requirement that a reversible reaction be at equilibrium is that:
- the concentrat...
- the concentrat...
Questions


Social Studies, 26.06.2019 22:00

Business, 26.06.2019 22:00



History, 26.06.2019 22:00

Social Studies, 26.06.2019 22:00

History, 26.06.2019 22:00

Mathematics, 26.06.2019 22:00


English, 26.06.2019 22:10

Chemistry, 26.06.2019 22:10

History, 26.06.2019 22:10



Mathematics, 26.06.2019 22:10




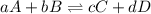
![K=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0438/0819/91a0a.png)


