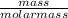Chemistry, 02.11.2019 23:31 pgjohnston001
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (iii) sulfide in moles would be produced in this reaction?
equation:
convert to moles
of iron:
convert to moles
of sulfur:
calculate the
limiting reagent:
solve the problem:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (ii...
Questions



Biology, 17.12.2020 22:50

Biology, 17.12.2020 22:50


Mathematics, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50

Physics, 17.12.2020 22:50

Social Studies, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50


Mathematics, 17.12.2020 22:50




Mathematics, 17.12.2020 22:50



Mathematics, 17.12.2020 22:50