Chemistry, 31.01.2020 13:56 sierravick123owr441
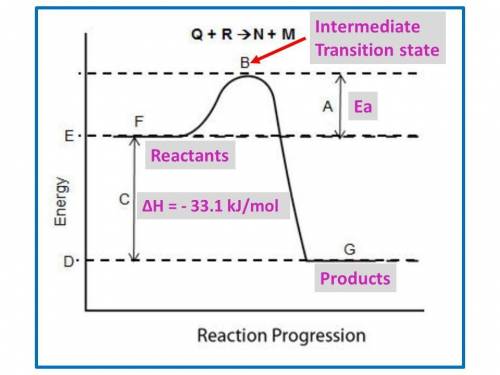
B. for the following questions, use the reaction no2(g) n2(g) + o2(g), with δh = –33.1 kj/mol and δs= 63.02 j/(mol·k).
i. draw a possible potential energy diagram of the reaction. label the enthalpy of the reaction.
ii. is the reaction endothermic or exothermic? explain your answer. (2 points)
iii. what is the gibbs free energy of the reaction at 25°c?
iv. is the reaction spontaneous or nonspontaneous at 25°c? explain your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
B. for the following questions, use the reaction no2(g) n2(g) + o2(g), with δh = –33.1 kj/mol and δs...
Questions

Mathematics, 20.10.2020 06:01

Chemistry, 20.10.2020 06:01


Biology, 20.10.2020 06:01




Mathematics, 20.10.2020 06:01



Mathematics, 20.10.2020 06:01

Mathematics, 20.10.2020 06:01

Chemistry, 20.10.2020 06:01

Arts, 20.10.2020 06:01

English, 20.10.2020 06:01


Mathematics, 20.10.2020 06:01

Mathematics, 20.10.2020 06:01


Mathematics, 20.10.2020 06:01