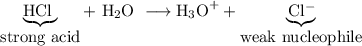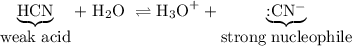Chemistry, 28.01.2020 17:02 elisechavez02
Explain why aldehydes and ketones react with a weak acid such as hydrogen cyanide but do not react with strong acids such as hcl or h2so4 (other than being protonated by them).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Explain why aldehydes and ketones react with a weak acid such as hydrogen cyanide but do not react w...
Questions


Mathematics, 15.05.2021 02:20

Mathematics, 15.05.2021 02:20






Mathematics, 15.05.2021 02:20

Biology, 15.05.2021 02:20



Spanish, 15.05.2021 02:20


Mathematics, 15.05.2021 02:30

Mathematics, 15.05.2021 02:30


Mathematics, 15.05.2021 02:30

English, 15.05.2021 02:30

Mathematics, 15.05.2021 02:30